CASE REPORT |
https://doi.org/10.5005/jp-journals-10070-7077 |
Metronidazole-induced Neurotoxicity: A Case Report
1,2Department of Neurology, Kuwait Hospital, Sharjah, United Arab Emirates
3–6Department of Internal Medicine, Kuwaiti Hospital, Sharjah, United Arab Emirates
Corresponding Author: Anandi Damodaran, Department of Neurology, Kuwait Hospital, Sharjah, United Arab Emirates, Phone: +971566073847, e-mail: anandimuthuraj@hotmail.com
How to cite this article: Damodaran A, Nayak D, Khan F, et al. Metronidazole-induced Neurotoxicity: A Case Report. Bengal Physician Journal 2022;9(1):19–21.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Metronidazole (MTZ) is an antibiotic from nitroimidazole group, commonly used for various medical and surgical indications especially anaerobic bacterial infection. Though usually safe, it can result in serious toxicity with prolonged use. It can affect both central and peripheral nervous system. Neurotoxicity presents as features of encephalopathy, cerebellar ataxia, dysarthria, and symmetrical sensory-motor polyneuropathy. Here, we are presenting our patient with liver abscess who received MTZ for 6 weeks and developed neurological side effects with cerebellar ataxia and dysarthria accompanied by MRI features—hyperintense signals in dentate nucleus consistent with toxicity and also features of symmetrical sensory-motor polyneuropathy. Cerebellar symptoms reversed with stopping the medication—however, his peripheral neuropathy had a static course with minimal recovery.
Keywords: Cerebellar signs, Dentate nucleus hyperintensity, Metronidaole toxicity, Peripheral neuropathy.
BACKGROUND
Metronidazole (MTZ) is an antibiotic from the nitroimidazole group, commonly used to treat anaerobic infections. Though common side effects are nausea, headaches, and metallic taste in the mouth, higher doses and/or prolonged use may cause severe neurological side effects including toxic peripheral neuropathy, cerebellopathy, encephalopathy, and seizures. MTZ easily penetrates the blood–brain barrier and can accumulate, leading to CNS toxicity. The duration of treatment with MTZ before encephalopathy develops varies between days to weeks and the cumulative dose. The occurrence of encephalopathy with toxic peripheral neuropathy is in fact a rare event. A high index of suspicion and a detailed neurological evaluation are needed to make a timely diagnosis of this potentially reversible phenomenon.
Here, we report a case of MTZ-induced neurotoxicity involving both central and peripheral nervous system with clinical as well as radiological findings, which resolved after stopping the medication.
CASE DESCRIPTION
A 69-year-old Filipino patient was admitted to our facility with complaints of fever and shortness of breath of a few days duration. Given the ongoing COVID pandemic, he was admitted to an isolation ward and started with work up to rule out COVID pneumonia. An initial CT scan of the chest and upper abdomen revealed a hepatic hydatid cyst with concomitant right-sided pleural effusion. He was started on broad spectrum antibiotics including MTZ at a dose of 750 mg IV TID. Pleural tap was performed, and 500 mL of the fluid was drained. Pleural fluid analysis was unremarkable. Patient remained febrile despite treatment, and the decision to undergo CT-guided cyst aspiration was taken. Seven hundred and fifty milliliters of pus was drained, and a pigtail catheter was left in situ. Patient continued to be on 750 mg MTZ IV TID. After completing 3 weeks on IV MTZ, he was shifted to oral MTZ for another 3 weeks and was discharged. However, within 2 weeks, the patient presented again to us in the ER with an acute onset of dysarthria and giddiness and ataxia. His clinical presentation suggested an acute posterior circulation stroke. CT brain being normal, we proceeded with MRI diffusion imaging which revealed symmetrical hyperintensity on T2W images involving bilateral dentate nuclei of the cerebellum (Fig. 1). As he also had hypotonic weakness of all four limbs with absent reflexes, nerve conduction studies were performed, which showed sensory and motor demyelinating polyneuropathy. His metabolic parameters were reportedly normal. MTZ-induced encephalopathy and neuropathy were suspected to be the cause of his acute neurological presentation, and his antibiotics were changed to suitable alternatives as per sensitivity. Culture of pus grew multidrug-resistant E. coli and histopathology confirmed the presence of Echinococcus cysts. After stopping the MTZ, the patient started showing signs of clinical neurological improvement. MRI brain repeated 4 days after the initial scan showed regression in the hyperintensity of the dentate nuclei (Fig. 2). Some baseline peripheral neuropathy, however, remained with no further worsening. Physiotherapy was initiated, and he was mobilized effectively and uneventfully before his discharge.
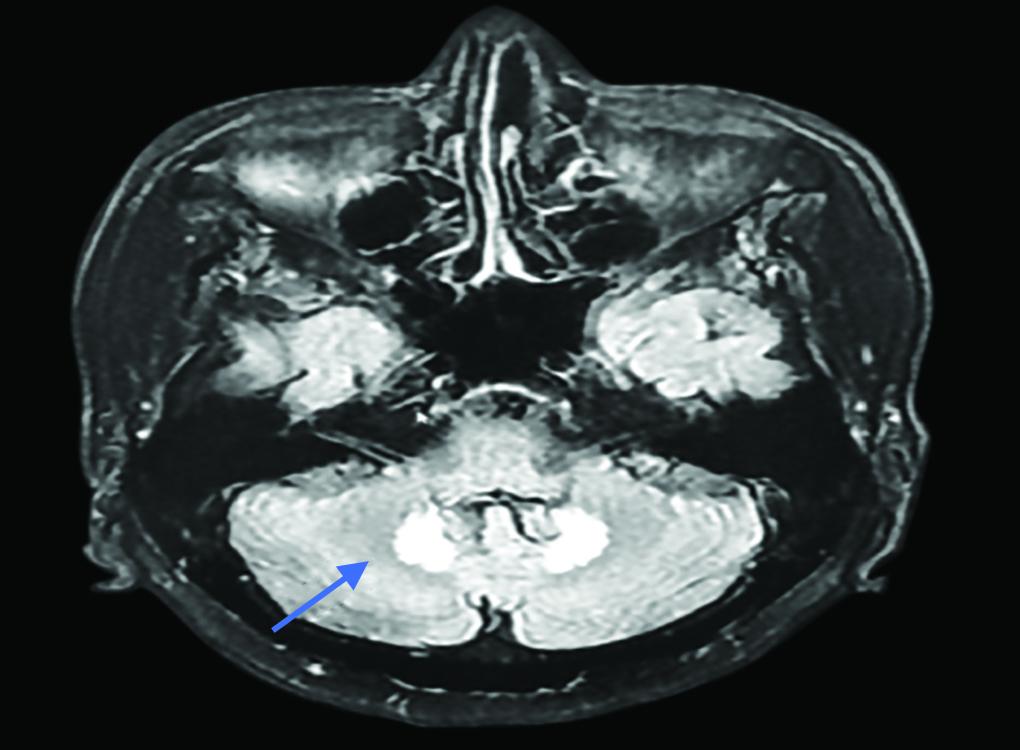
Fig. 1: Axial flair image showing hyperintense lesion in bilateral dentate nuclei

Fig. 2: Follow-up axial flair MRI showing regression of hyperintensity in dentate nucleus
DISCUSSION
MTZ, being one of the most used antimicrobials, has the potential to cause serious neurological side effects—in both long term and short term. First case of MTZ-induced neurotoxicity was reported in 1977 in a 19-year-old woman with features of encephalopathy. Subsequently numerous case reports have been published. In a recent systematic review of 136 reported cases of MTZ-induced neurotoxicity, the duration of treatment with the antibiotic before the onset of CNS dysfunction varied from 14 to 52.5 days.1 Sorensen et al. also showed that of the 136 patients, 119 had either partial or complete resolution of CNS symptoms after stopping MTZ. Twelve of the 136 patients had unfavorable outcomes including death or significant persistent neurological deficits. However, all these 12 patients had previous premorbid conditions including organ failure or malignancy.
MTZ affects both central and peripheral nervous system. In peripheral nervous system, it causes slowly progressive symmetric distal sensory-motor polyneuropathy with severe paresthesia, leading to permanent disability. In central nervous system, it causes encephalopathy and cerebellar dysfunction with features of dysarthria, ataxia, dysmetria, and nystagmus.2 Two-thirds of patients with CNS complications attributable to MTZ improve eventually on stopping the drug.
Pathogenesis of MTZ toxicity is unknown. MTZ crosses the blood–brain barrier easily, and its intermediate metabolites bind to DNA and RNA of neuronal cells and induce generation of superoxide radical which causes axonal swelling.3 Another leading theory is that the axonal swelling is caused by vasogenic edema, possibly due to impairment in vitamin B1 activity given MTZ’s conversion of a thiamine analog in vivo. Whether MTZ-induced neurotoxicity is dose-dependant or idiosyncratic is not clear. Studies have shown that steroid treatment may hasten recovery, possibly due to its effect on reducing the vasogenic edema that contributes to MTZ’s neurotoxicity.4
Ahmed et al. was the first to describe imaging finding of MTZ toxicity. He reported symmetrical changes in corpus callosum and cerebellum including deep grey matter. He proposed axonal swelling and increased water content for the cause of this change.5
Chandak et al. reported a similar case of liver abscess treated with MTZ presented with cerebellar symptoms and the patient MRI showed hyperintense signal changes in dentate nuclei which resolved completely within 3 days.6 Unlike our case, however, there was no peripheral neuropathy. Hwang et al., in 2012, reported a case of MTZ toxicity who presented with both cerebellar toxicity and peripheral neuropathy. The imaging in their patient showed hyperintense signals in superior olivary nuclei of the dorsal pons, splenium of corpus callosum in addition to dentate nuclei. The cerebellar symptoms in their patient improved without much improvement of the peripheral neuropathy.7
Peng et al., in 2020, and Phyu et al., in 2019, also described a similar case with peripheral neuropathy and encephalopathy with MRI showing corpus callosum involvement and bilateral dentate nuclei involvement, respectively. Their patient also showed regression of lesion in MRI after stopping MTZ—however, peripheral neuropathy showed little improvement.8,9
Similar to their cases, our patient presented with dizziness, dysarthria, and features of peripheral neuropathy. Patients’ symptoms improved on stopping the medication. MRI brain repeated after 4 days also showed regression of cerebellar hyperintensity, whereas the peripheral neuropathy showed little improvement.
Lesions of MTZ-induced encephalopathy are typically located at the cerebellar dentate nuclei, midbrain, dorsal pons and medulla, inferior colliculus, and splenium of the corpus callosum bilaterally and symmetrically.10 The differential diagnosis of imaging findings in MRI includes demyelinating diseases and metabolic, infectious, and inflammatory processes. Despite many diagnostic possibilities, cerebellar toxicity due to MTZ is a diagnosis which can be easily overlooked, especially in patients with comorbidities.
CONCLUSION
High index of suspicion is needed to diagnose these cases especially when patients present with new-onset neurological symptoms after initiation of MTZ. MRI is an important tool in evaluation of these cases. In case of doubt, it is better to stop the medication and change the antibiotic to see improvement.
REFERENCES
1. Sorensen CG, Karlsson WK, Amin FM, et al. Metronidazole-induced encephalopathy: a systematic review. J Neurol 2020;267(1):1–13. DOI: 10.1007/s00415-018-9147-6.
2. Daneman N, Cheng Y, Gomes T, et al. Metronidazole-associated neurologic events: a nested case-control study. Clin Infect Dis 2021;72(12):2095–2100. DOI: 10.1093/cid/ciaa395.
3. Frasca D, Dahyot-Fizeier C, Adier C, et al. Metronidazole and hydroxy metronidazole central nervous system distribution: Microdialysis assessment of brain extracellular fluid concentrations in patients with acute brain injury. Antimicrob Agents Chemother 2014;58(2):1019–1023. DOI: 10.1128/AAC.01760-13.
4. Li L, Tang X,Li W, et al. A case of methylprednisolone treatment for metronidazole-induced encephalopathy. BMC Neurol 2019;19(1):49. DOI: 10.1186/s12883-019-1278-6.
5. Ahmed A, Loes DJ, Bressler EL. Reversible resonance imaging findings in metronidazole-induced encephalopathy. Neurology 1995;45:588–589. DOI: 10.1212/wnl.45.3.588.
6. Chandak S, Agarwal A, Shukla A, et al. A case report of metronidazole induced neurotoxicity in liver abscess patient and the usefulness of MRI for itsdiagnosis. J Clin Diagnostic Res 2016;10(1):TD06–TD07. DOI: 10.7860/JCDR/2016/14900.7031.
7. Hwang GH, Sim YJ, Jeong HJ, et al. Metronidazole induced encephalopathy with peripheral polyneuropathy in patient with spinal cord injury. Korean J Spine 2012;9(1):44–48. DOI: 10.14245/kjs.2012.9.1.44.
8. Peng Q, You Q, Zhang J, et al. Isolated involvement of corpus callosum in metronidazole-induced encephalopathy with concomitant peripheral neuropathy. A case report. Medicine (Balimore) 2020;99(20):e20198. DOI: 10.1097/MD.0000000000020198.
9. Phyu H, Edmond MB, Kobayashi T, et al. Metronidazole-induced encephalopathy. ID Cases 2019;18:e00639. DOI: 10.1016/j.idcr.2019.e00639.
10. Kim E, Na DG, Kim EY, et al. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol 2007;28(9):1652–1658. DOI: 10.3174/ajnr.A0655.
________________________
© The Author(s). 2022 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.